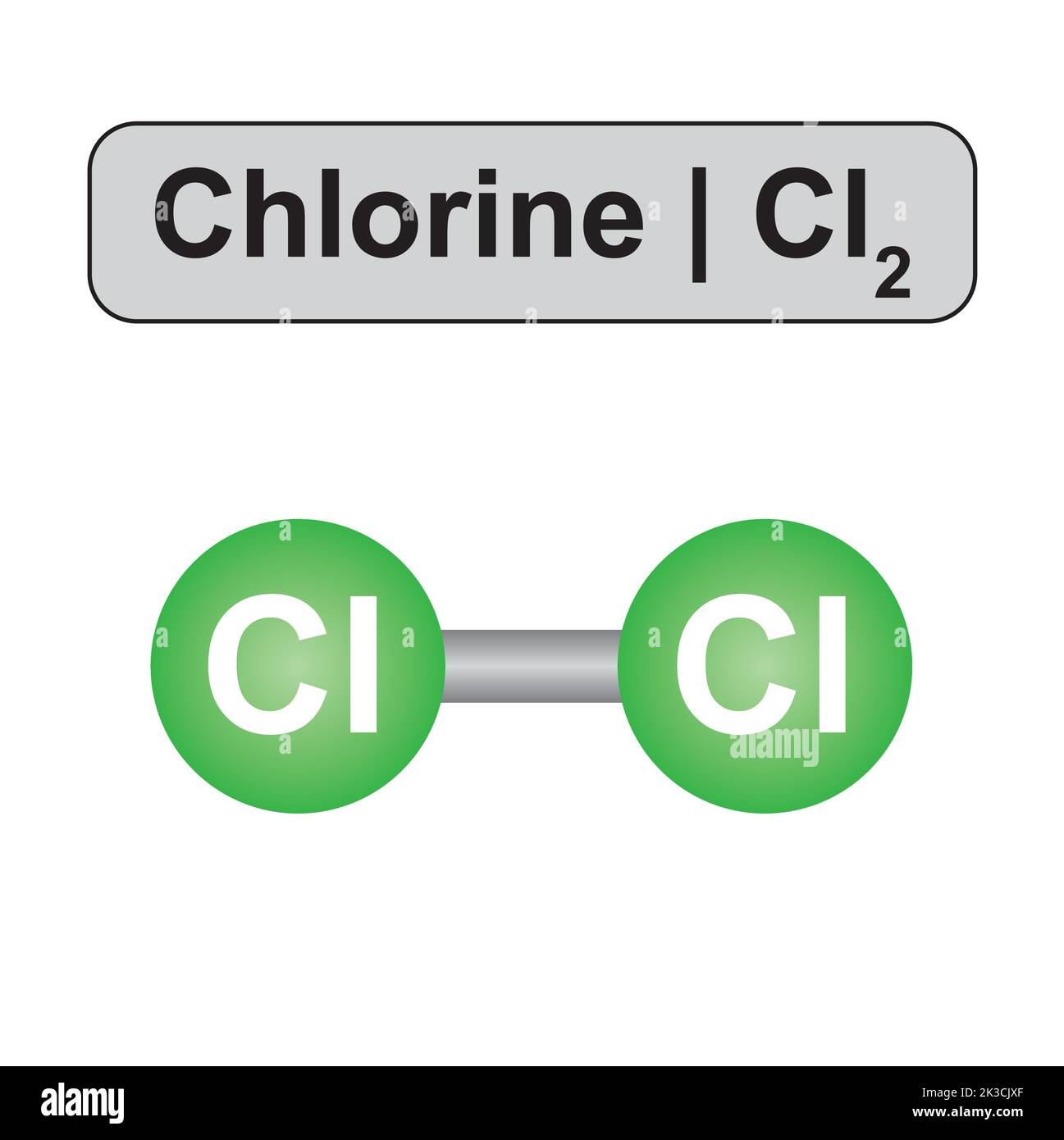
Chlorine Gas Diatomic . this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by the chemical symbol cl, it. diatomic elements are pure elements that form molecules consisting of two atoms bonded together.
from www.alamy.com
like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by the chemical symbol cl, it. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals.
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock
Chlorine Gas Diatomic Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by the chemical symbol cl, it.
From www.youtube.com
Chlorine 3D Diatomic Elements YouTube Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by the chemical symbol cl, it. diatomic elements are pure elements that form molecules consisting. Chlorine Gas Diatomic.
From www.kindpng.com
Sensors Chlorine Info Support Homonuclear Diatomic Molecule Hcl, HD Chlorine Gas Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by the chemical symbol cl, it. diatomic elements are pure elements that form molecules consisting. Chlorine Gas Diatomic.
From www.bartleby.com
Diatomic Gas bartleby Chlorine Gas Diatomic Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Chlorine Gas Diatomic.
From brainly.com
The diatomic molecule of chlorine, cl2, is held together by a(n Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Denoted by. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by. Chlorine Gas Diatomic.
From geteducationbee.com
Diatomic Elements Best Definition, Example & More Get Education Bee Chlorine Gas Diatomic Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by the chemical symbol cl, it. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by. Chlorine Gas Diatomic.
From www.pinterest.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split. Chlorine Gas Diatomic.
From www.slideserve.com
PPT Molecular Compounds and Covalent Bonds PowerPoint Presentation Chlorine Gas Diatomic this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Denoted by. Chlorine Gas Diatomic.
From www.vedantu.com
Which of the following is a diatomic gas?(A) Hydrogen(B) Oxygen(C Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split. Chlorine Gas Diatomic.
From www.alamy.com
Green chlorine gas Stock Vector Images Alamy Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by the chemical symbol cl, it. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. this radical reaction is initiated by the use of sunlight or ultraviolet light to split. Chlorine Gas Diatomic.
From www.sarthaks.com
In a crude model of a rotating diatomic molecule of chlorine `(Cl_2 Chlorine Gas Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. Denoted by. Chlorine Gas Diatomic.
From www.scienceabc.com
Diatomic Molecules Definition, Explanation And Examples Chlorine Gas Diatomic this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by the chemical symbol cl, it. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by. Chlorine Gas Diatomic.
From sciencenotes.org
What Are the 7 Diatomic Elements? Definition and List Chlorine Gas Diatomic like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \(cl_2\) rather than cl. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. Denoted by. Chlorine Gas Diatomic.
From www.alamy.com
Chlorine is a yellowgreen gas under standard conditions, where it Chlorine Gas Diatomic Denoted by the chemical symbol cl, it. this radical reaction is initiated by the use of sunlight or ultraviolet light to split diatomic chlorine into two radicals. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring. Chlorine Gas Diatomic.